Identify the pair of elements undergoing oxidation and reduction by checking oxidation states. Mn 2 bio3 ae mno4 bi 3 mno4 s2o3 2 ae s4o6 2 mn 2.
 Balancing Redox Reactions Worksheet Reactions Worksheet Answers
Balancing Redox Reactions Worksheet Reactions Worksheet Answers Cr 2o 7 2 cr3 5.
Balancing redox reactions worksheet. Balancing redox reaction equations is a skill which combines chemical knowledge common sense and intuition. Balancing redox reactions worksheets 1 2 with answers free download as pdf file pdf text file txt or read online for free. Balancing oxygen and hydrogen in basic redox reactions sometimes can be difficult because both oh and h 2 o contain both elements.
Balancing redox reactions author. Zn no 3 zn2 nh 4 3. Oxidation reduction balancing additional practice problems acidic solution 1.
If the redox reaction was carried out in basic solution ie. Microsoft word oxidation reduction extra practicedoc author. 3au aq i aq au s i 2 s 4.
S2 aq i 2 s so 4. Worksheet 5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. Al s h 2so 4 aq al 2so 4 3 aq h 2 g 3.
Todd helmenstine created date. Some of the worksheets displayed are chapter 20 work redox redox practice work work 25 chemistry 30 work key review work on balancing redox equations work 1 redox reactions balancing redox reactions academic resource center. The steps for balancing redox reactions in basic solution are.
Showing top 8 worksheets in the category redox. Cr 2o 7 2 c 2h 4o c 2h 4o 2 cr 3 4. Balance the following redox reactions 1.
Balancing redox reactions in basic solution. Ag no 3 ag no 2. Balancing redox reactions worksheet 1 balance each redox reaction in.
There are many methods available for balancing redox reactions a number have been brought to your attention including the one that follows. H 2 co 3 b. Alkaline conditions then we have to put in an extra step to balance the equation.
Choose a method and complete questions 12 13. So 2 g hno 2 aq h 2so 4 aq no g 2. Znoh 4 2 d.
Determine the oxidation number of the elements in each of the following compounds. A trick to get around this is to balance any troublesome half reaction or the entire redox reaction first as if it were in acid using h and h 2 o.
 Balancing Redox Reactions Worksheet For 10th 12th Grade Lesson
Balancing Redox Reactions Worksheet For 10th 12th Grade Lesson  Chemical Equations And Reactions Worksheet Balancing Answers
Chemical Equations And Reactions Worksheet Balancing Answers  H Chem Keys
H Chem Keys  Picture The Study Buddy Redox Reactions Balancing Equations
Picture The Study Buddy Redox Reactions Balancing Equations  Balancing Redox Reactions
Balancing Redox Reactions  Balancing Redox Equations Worksheet
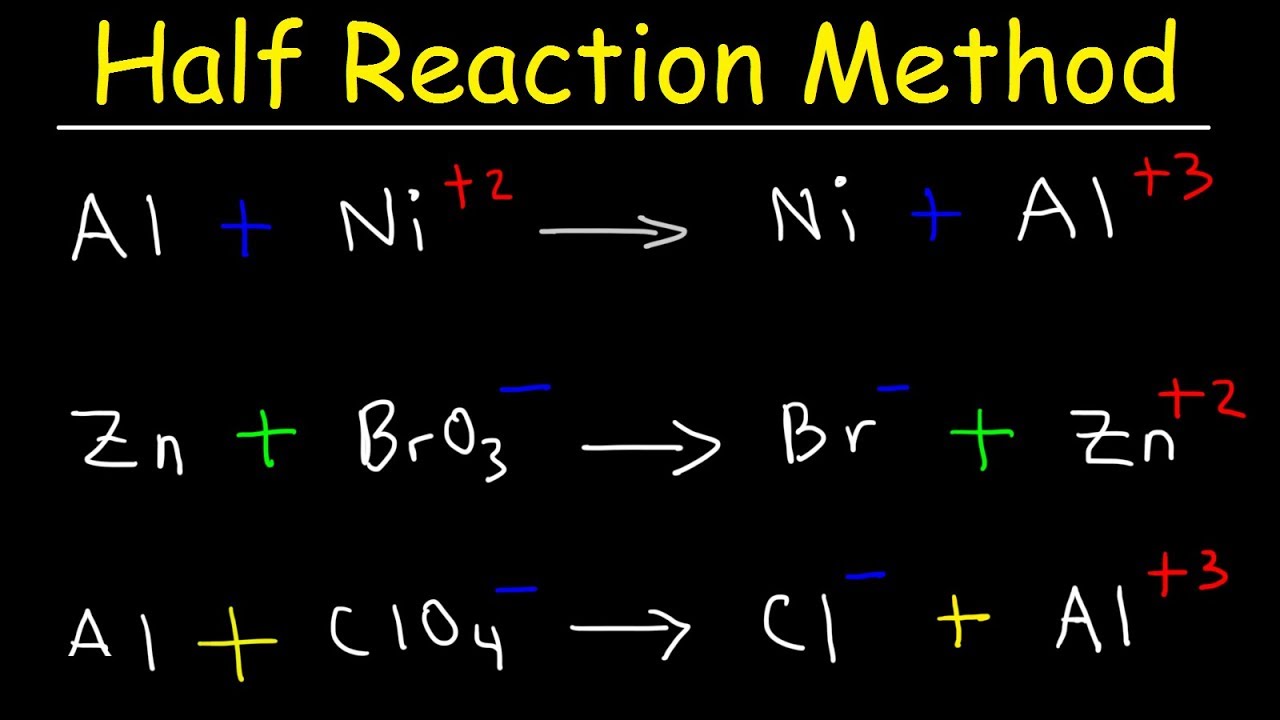
Balancing Redox Equations Worksheet  Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry
Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry 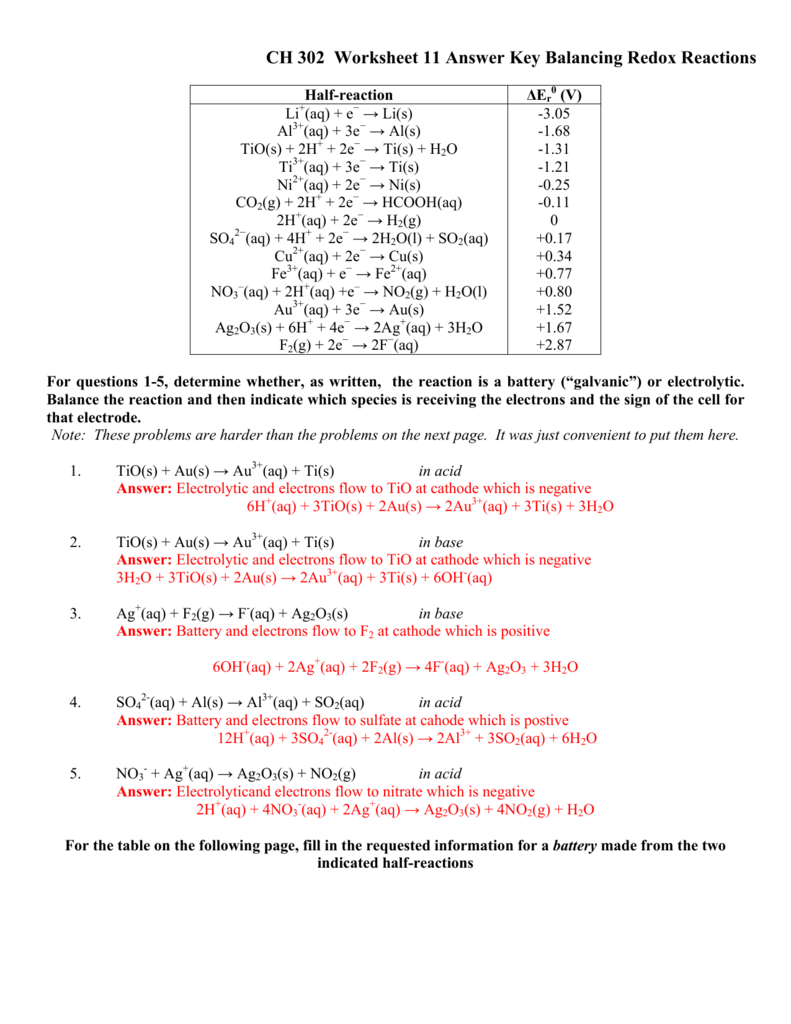 Ch 302 Worksheet 11 Answer Key Balancing Redox Reactions
Ch 302 Worksheet 11 Answer Key Balancing Redox Reactions  Steps For Balancing Half Reactions In Basic Solution Acid Base How
Steps For Balancing Half Reactions In Basic Solution Acid Base How
0 comments